A key problem in sensory processing is to find out which information from the outside world belongs together to form a single object. In visual scene analysis, this task often boils down to recognizing the boundaries between different objects. A conspecific, for example, might have a color that is very different from that of the vegetation in the background. Since visual objects practically always consist of one spatially connected entity, determining boundaries of an object is often enough to isolate it from the background.
In auditory processing, this task is similar, but in some respects more difficult. The question that needs to be answered is: What is the boundary of an auditory object? In visual processing, spatial seperation of objects directly translates to spatially seperated responses on the retina. In contrast to this, the auditory system has only two spatially separated input channels (the ears at both sides of the head) instead of millions of retina cells. Before an auditory image of the environment can be formed, some processing steps are necessary. We focus here on two projects of our group: monaural echo suppression and identification of periodic signal features.
Any biological system processing acoustic signals has to cope with reflections interfering with the original signal. Hence, a neural mechanism suppressing the confusing information of echoes is advantageous for sound perception.
Echoes arriving less than about 20 ms after the original signal usually are not perceived consciously. A simple experiment shows that echo suppression is in part binaural and in part monaural. In a large room, for example a lecture room, one can perceive the otherwise suppressed echoes by covering one ear. In a small room, where the echoes are faster, this does not work. Thus, neuronal echo suppression consists of a slower, binaural mechanism using both ears and a faster, monaural mechanism using only the cues one ear can provide. Since 1963 (Harris et al.) the cochlear nucleus, the first neural node after cochlea and auditory nerve, was supposed to be involved in echo suppression. The aim of our research was to give a quantitative answer to the question to which extent an inhibitory loop found in the cochlear nucleus in 1990 (Wickesberg and Oertel) can account for the phenomenon of monaural echo suppression.
The model
According to the biological evidence, the model consisted of three different populations of neurons: the neurons of the auditory nerve (AN), of the dorsal part of the cochlear nucleus (DCN), and of the anteroventral part of the cochlear nucleus (AVCN). In order to keep the model as simple as possible, we have used identical parameters for the neurons in the AVCN and DCN. The cell parameters for our generic neurons were taken from an article by Wickesberg and Oertel which mainly studied bushy cells. The connections between the different populations have also been modeled to match biological findings. Each neuron of the auditory nerve is connected excitatorily to one neuron of the DCN and to one neuron of the AVCN while the neuron in the AVCN is receiving inhibitory input from the cell in the DCN. Every three neurons that are connected as described above form a neuronal triad receiving input from only one frequency channel.
In a first step the neurons have been modeled as Poisson neurons, a simple neuron model which is analytically tractable. As a second step more realistic Spike-Response neurons, a generalization of the Leaky-Integrate-and-Fire neuron, have been used.
It is important to note that the behavior of the model remains essentially unchanged by varying the parameters of the model neurons. The parameters we have chosen were taken from publications by Wickesberg, Oertel and Wu, but also for different sets of parameters the behavior of the model remained stable. The robustness of our results show that the neural circuitry discovered by Wickesberg and Oertel does per se have the properties several authors speculated to be found in the cochlear nucleus. This means that we can indeed, as Wickesberg and Oertel proposed, assume monaural echo suppression to be at least one of the purposes of the cochlear nucleus. A click quickly following another click will under natural circumstances most often be an echo. In this sense, the cochlear nucleus can be regarded as the site of monaural echo suppression.
It is known that echoes occurring 2-20 ms after the initial signal are suppressed in the central nervous system. A model for binaural echo suppression that works for echoes up to 20 ms has been proposed previously. It has a minimal suppression time of 5 ms, which means it is not capable of a suppression occurring in less than 5 ms. So, the model presented here with a suppression time from about 2-4 ms can complete the picture.
An important acoustic binding cue is periodic modulation of the input signals. Therefore, a common problem in auditory processing is the recognition and identification of such fluctuations. It has been known for a long time that complex sounds composed of many different frequencies may nevertheless be perceived as one single acoustic entity, having one clear pitch. This pitch often does not correspond to any of the frequency components present in the signal, but rather to the temporal structure of the signal envelope. Such temporal structure is generally believed to play an essential role in object identification and recognition.
Since temporal cues in the input signal cannot be resolved using the cochlea, there must be a neuronal mechanism to identify periodic signal features. Although several neuronal models have been proposed that try to deal with this problem a detailed mathematical study of such models has never been undertaken. In recent work, we have analyzed a simple periodicity detection model which is based on a network of neuronal delay lines. We have been able to pin down the essential parameters that limit performance of such networks and to understand why these networks function in the way they do.
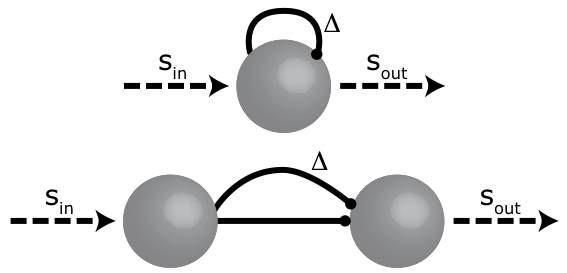 The basic network that underlies the detection of signal periodicity is a set of neuronal delay lines. As shown in the figure, such a delay line can be implemented by a feedback connection or by a feedforward connection. Each set of neurons that are connected in such a way have a particular delay (Delta), that roughly corresponds to the inverse period (1/T) of the periodicity. If, for example, a signal is amplitude modulated with a frequency of 100 Hz, the input signal Sin will increase and decrease 100 times per second. If the length of the delay line is 10 ms -corresponding precisely to the period of the input fluctuation- the output signal will be strong since the direct and the delayed input work together. If the length of the delay line does not match the periodic features of the signal, the output will be much weaker.
The basic network that underlies the detection of signal periodicity is a set of neuronal delay lines. As shown in the figure, such a delay line can be implemented by a feedback connection or by a feedforward connection. Each set of neurons that are connected in such a way have a particular delay (Delta), that roughly corresponds to the inverse period (1/T) of the periodicity. If, for example, a signal is amplitude modulated with a frequency of 100 Hz, the input signal Sin will increase and decrease 100 times per second. If the length of the delay line is 10 ms -corresponding precisely to the period of the input fluctuation- the output signal will be strong since the direct and the delayed input work together. If the length of the delay line does not match the periodic features of the signal, the output will be much weaker.
A collection of such delay lines, all with a different temporal length, can analyse an input signal and filter out periodic components. We have shown that the temporal precision of the signals is conserved. That means that once auditory objects have been identified through the delay-line system, it is possible to further process the signal without loss of precision. In particular, localization of an auditory object using interaural time differences is possible.
It has often been suggested that auditory objects are identified by simply isolating all auditory input that comes from one spatial location. A problem with this approach is that if multiple input sources are present, the auditory signals of all those sources will interfere. This effect makes is very difficult to determine the location of the sources. In our model, object identification takes place before object localization. As the temporal precision does not detoriate during this processing mechanism, object localization is still possible after attention has been focussed on one particular object, thus filtering out distracting information from other sound sources.
- Bürck M., van Hemmen J.L. Modeling the cochlear nucleus: A site for monaural echo suppression? J. Acoust. Soc. Am. 122:2226-2235 (2007)
- Friedel P., Bürck M., van Hemmen J.L. Neuronal identification of acoustic signal periodicity Biol. Cybern. 97:247-260 (2007)
- Harris G.G., Flanagan J.L., Watson B.J. Binaural interaction of a click with a click pair J. Acoust. Soc. Am. 35:672-678 (1963)
- Wickesberg R.E., Oertel D., Delayed, frequency- specific inhibition in the cochlear nuclei of mice: A mechanism for monaural echo suppression J. Neurosc. 10:762-1768 (1990)
- Zahn T.P., Neural achitecture for echosuppression during sound source localization based on spiking neural cell models Ph.D. thesis, Technische Universität Ilmenau (2003).
- Pecka M., Zahn T.P., Saunier-Rebori B., Siveke I., Felmy F., Wiegrebe L., Klug A., Pollak G.D., Grothe B. Inhibiting the inhibition: a neuronal network for sound localization in reverberant environments J. Neurosci. 27:1782-1790 (2007)



